Aluminum phosphate is a white crystalline powder A colorless liquid in aqueous form with a foul odour. Barium phosphate Ba3PO42 manganeseIV fluoride MnF4 potassium antimonate K3SbO4 chromous sulfite CrSO3 silver sulfate Ag2SO4 plumbous hypochlorite PbClO2.

How To Write The Formula For Barium Phosphate Youtube
Barium is a group 2 element.

. The formula for barium phosphate is Ba3 PO42. Both ions are bound by one ionic bond. Molar mass of Li3PO4 115794361 gmol Convert grams Lithium Phosphate to moles or moles Lithium Phosphate to grams.
Barium phosphate is bonded to an ionic. The density of Aluminum phosphate is 256 gcm 3 in solid-state. The barium oxide chemical formula is BaO.
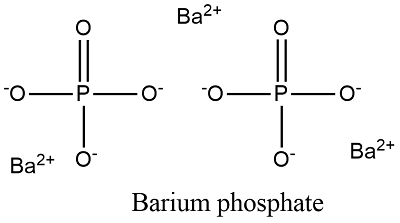
What is the ionic formula for barium and phosphate. Diphosphate the barium phosphate formula is written as Ba 3 PO 4 2. Ba3 PO42 What is the correct formula for the compound formed by Ca2 and NO2.
E-mail to a friend. 5 rows The formula for barium phosphate is Ba3 PO42. Ionic Compound Formula Writing Worksheet.
Group 2 elements form cations with a 2 charge. 251 rows Calcium Phosphate. The chemical formula for barium phosphate is Ba 3 PO 4 2.
The balanced equation is 3 AgNO3 aq Na3PO4 aq equals Ag3PO4 s 3 NaNO3 aq. This problem has been solved. FORMULA OF IONIC COMPOUNDS D.
It has a very high coefficient of thermal expansion and melting point. The geometry of the molecule is octahedral. Barium phosphate Ba3 PO42 - PubChem.
Name Chemical Formula Ammonium phosphate NH43PO4 Mercury II silicate HgSiO3 Lithium phosphite Li3O3P Manganese II borate Mn3BO32 Barium nitrate. What is the formula for an ionic compound made of barium. Copy this to my account.
Fill each of the boxes with the formula of the appropriate ionic compound. Barium phosphate would be Ba3PO42. 100 4 ratings well we know that Barium ions are having.
In this video well write the correct formula for Barium phosphate Ba3PO42To write the formula for Barium phosphate well use the Periodic Table and foll. Ionic Compounds Naming and Formula Writing. Physical Properties of Aluminum Phosphate.
Therefore the formula is three barium ions to two phosphate ions. What results when two or more atoms share electrons equally. Does barium react with phosphorus.
It is used to make a sodium and barium polyphosphate combination which is one of the components of hot glasses and other special-purpose glasses. Write the formula for each polyatomic ionic compound shown below. - Answers The barium cation is Ba2.
Barium Nitrate And Potassium Phosphate What is the balanced equation for barium nitrate and. The molecule is formed by one barium cation Ba2 and one oxide anion O2-. What is the correct formula for an ionic compound that contains barium ions and phosphate ions.
Ans- The IUPAC name given to the barium phosphate is barium2. Calcium nitrite Ca NO22 PubChem. Which of these are ionic compounds.
2 as shown. State Some of the Physical Features of the Salt. View the full answer.
Learn vocabulary terms and more with flashcards games and other study tools. What is the formula for Ba3 PO4 2. Barium phosphate is bonded to an ionic bond because the bond is created by giving or taking electrons.
Three barium ions each with a charge of 2 would give us six positive charges while two phosphate ions each with a charge of 3 would give us six negative charges. For the following compounds give the formulas and the molar masses. More 209 People Used More Info Visit site.
Start studying Ionic Compound - Formula. FORMULA OF IONIC COMPOUNDS C. The molar mass is 15333 gmol.
The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl. Write chemical formulas for the compounds in each box. This is the best answer based on feedback and ratings.
Formula 21 sodium phosphide Na 3P 22 magnesium nitrate MgNO 32 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3PO 43 25 ammonium sulfate NH 42SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2S3 28 beryllium chloride BeCl 2. Home Subjects Math Science History. The Melting point of Aluminum phosphate is 1800C.
Common Compounds of Barium Ba 2. The names are found by finding the intersection between the cations and anions. The formula for barium phosphate is Ba3 PO42.
The Aluminum phosphate is decomposed at its Boiling point. See the answer See the answer done loading. 5 rows Barium and phosphorus form an ionic compound with the formula Ba3P2.
Three barium ions 2. The phosphate anion is PO4 3-. The formula for the barium ion is Ba2.

0 Comments